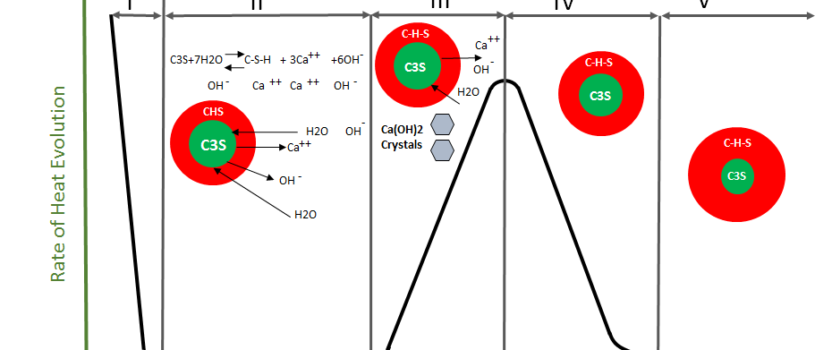
Kinetics of Cement Hydration
I. Pre-induction period (first minutes):
- Dissolution and saturation, contributing Ca2+ and SO42-.
- C-S-H forms on the surface of dissolving C3S, thus an increase of the Ca2+ concentration in the liquid phase occurs.
- Precipitation of CH leads to the dormant period.
- C3A dissolves, and reacts with SO42- to form AFt, which forms a surface barrier.
- C4AF also reacts to form AFt.
- Only very small % of C2S reacts at this stage.
II. Induction (dormant) period (first few hours):
- CH concentration in the liquid phase reaches a maximum and then starts to decline.
- Concentration of SO42- remains constant as the amount consumed due to AFt formation is balances by the amount dissolved from gypsum.
III. Acceleration stage (3 – 12 hours after mixing):
- Nucleation and growth of C-S-H and CH occurs.
- C2S also starts hydrating substantially.
- Ca2+concentration in the liquid phase declines as Ca(OH) 2 starts precipitating.
- SO42- concentration starts to decline with increasing AFt formation,
IV. Post-acceleration period:
- Slow down due to decline in non-reacted material, and because the process becomes diffusion controlled.
- The contribution of C2S increases steadily, leading to a decline in the rate of formation of CH.
- Consumption of SO42- leads to a conversion of AFt to AFm.
V. Diffusion Period (Steady-state period):
- During this stage, the diffusion is so slow that the rate of hydration is controlled solely by the rate of diffusion.
- As the thickness of the layer continues to grow, the rate of diffusion continues to decrease until there is no more C3S to hydrate.

1 Comment
Leave your reply.